


|
Pharma Lifter Washing Station |


 |
VTD series Pharma Lifter is mainly used in the pharmaceutical industry for transferring and charging powder and granules. It can be used along with such main equipments as bin blender, tablet press, capsule filling machine and so on. Applying the VTD series Pharma Lifter has optimized the producing flow, avoided dust and cross pollution. Its the desirable transferring equipment in pharmaceutical industry for solid dosage sector. Meanwhile, it can be widely used in such areas as raw medicine material industry, chemical industry, foodstuff industry, etc. VTD series Pharma Lifter consists of fixed chassis, post, hydraulic system, turnover structure, horizontal gyration structure and skid appliance. To start to work, switch on the operation handle on the control panel, the bin is lifted to the required height; then gyrate to the post horizontally. Switch on the skid appliance on the fixed chassis by foot and fix the outlet of the bin with the inlet of the next processing equipment. Open the butterfly valve on the bin, materials flow out fluently into the next equipment. It has avoided dust and cross pollution and optimized the producing flow. Its is conformity with GMP requirements.
Its made of Austenitic stainless steel. There is no dead angle,
no concave/convex face, no screw on the surface. Its easy to
wash. Tailor-made butterfly valve with no dead angle, remnant, and
appliance against wrong way operation is easy to dismantle and
wash. All the crafts are finished in the same container to avoid
the frequent charging, shifting and transferring. This has avoided
dust and cross pollution and materials layering, optimized the
producing flow. VTD series Pharma Lifter is the brand-new
successfully developed type by our company widely researching,
absorbing and digestion foreign updated models. Its
authenticated by the Provincial Science Committee to be of
reasonable design, compact structure, steady performance,
beautiful form, easy to wash; main technical level reaches to the
world similar products of the 1990s Its in conformity with
GMP requirements.
| |
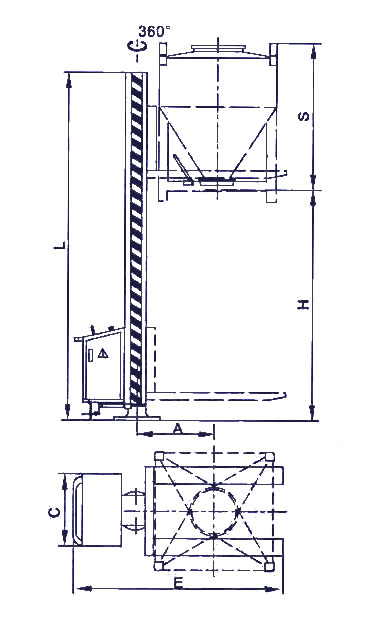
| Parameters\Type | VTD - 600 | VTD - 800 | VTD - 1000 | VTD - 1200 | VTD - 1500 | VTD - 1800 | VTD - 2000 |
| H | 2500 | 2500 | 2500 | 2500 | 2500 | 2500 | 2500 |
| S | 1300 | 1340 | 1375 | 1525 | 1625 | 1770 | 2025 |
| L | 3400 | 3600 | 3600 | 3800 | 3800 | 3800 | 3800 |
| A | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 | 1100 |
| E | 1940 | 2140 | 2140 | 2140 | 2140 | 2140 | 2140 |
| C | 750 | 750 | 750 | 750 | 750 | 750 | 750 |
| Power (kw) | 2.2 | 2.2 | 3 | 3 | 3 | 3 | 3 |
| Siut Bin (L) | 400 600 | 600 800 | 800 1000 | 1000 1200 | 1200 1500 | 1500 1800 | 1800 2000 |
| Home | | | Contact Us | | | About Us | | | Price List | | | Warranty Terms | | | Privacy Statement | | | Copyright |
Copyright 1997-2008 Vanguard Pharmaceutical Machinery, Inc. All rights reserved.